Transportation is the largest source of climate-warming emissions & air pollution. To combat this, we must enhance infrastructure for low emission vehicles and transition to electric power. We must also understand the needs of electrification. This article addresses four key questions about EV batteries, helping you determine the best battery for E-mobility.
The number of people buying electric vehicle is rising day by day and that is a good news. It is good news because we are making progress in the transport electrification journey. Many governments and employers are replacing their gas-powered trucks, vans, and buses with those powered by electricity. However, to speed up EV adoption, we’ll need to improve the ways we mine, process, and assemble the materials that go into an EV battery. It is important if you are someone who want to establish a lithium-ion battery production plant. You can gain knowledge from here, this article and when you make up you mind to set-up your battery plant, Semco can help you. Semco helps EV makers to change lives by helping them set-up their battery assembly plant. How and what we do is something you can find out by contacting us. Understanding how an EV battery works can help policymakers make informed decisions, help people choose an EV that best meets their needs. It may also guide investor resources, and equip the private and public sectors with the tools they need to develop efficient and effective technologies.
What Kind of Batteries Should EVs Use?
Most electric vehicles are powered by lithium-ion batteries and regenerative braking, A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. In comparison with other commercial rechargeable batteries, Li-ion batteries are characterised by a higher specific energy, higher energy density, higher energy efficiency, a longer cycle life, and a longer calendar life. Also, a noteworthy improvement in lithium-ion battery properties after their market introduction in 1991. Within the next 30 years, their volumetric energy density increased threefold while their cost dropped tenfold.
There are several types of lithium-ion batteries, with lithium Nickel Manganese Cobalt Oxide (NMC), and Lithium Iron Phosphate (LFP) batteries being the most common ones used in EVs. Like all batteries, both NMCs and LFPs have their strengths and shortcomings:
NMC versus LFP
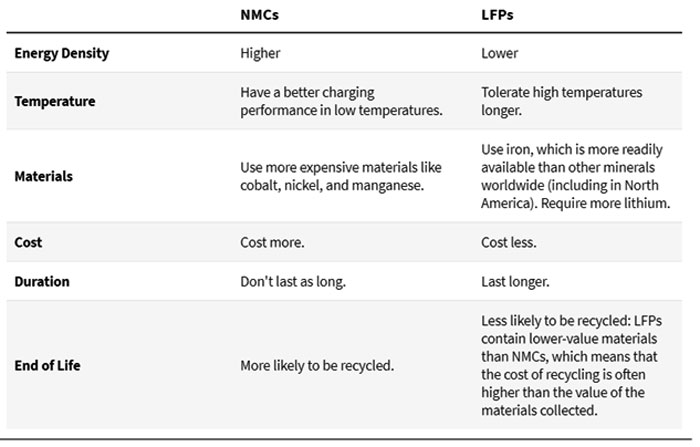
All batteries have their unique chemistry, each of which has its trade-offs. There’s no overall “best” battery for all EVs.
Why Are Lithium-ion Batteries Used in EVs?
Lithium-ion batteries are used in EVs because they:
- Have high energy density to store a relatively large amount of electrical energy in a smaller and more lightweight package than other battery technologies.
- Perform well at high temperatures and can withstand low temperatures without being damaged.
- Have a low self-discharge rate, meaning that the battery holds its energy well even if it’s not used for days or weeks.
- Are able to withstand many charge cycles while retaining almost all of their original capacity.
How Do Lithium-ion Batteries Work?

Lithium-ion batteries, like all batteries, store energy and convert it to electrical energy when in use. This electricity is produced by the movement of electrons, which are small particles with a negative charge that are found in all atoms.
Chemical reactions within the battery move these electrons from one electrode to another. There are two electrodes in a battery: the anode (a negative electrode) and the cathode (a positive electrode). Electrons start off in the anode and then move to the cathode through an electrolyte medium, which can be either liquid or solid.
When the battery is in use, the electrons move from the anode electrode to the cathode electrode; when the battery is charging, they move from the cathode to the anode.
To explain this movement, imagine that an electron is a person taking a bus to the grocery store. The anode is the person’s home while the cathode is the grocery store. The electrolyte medium is the bus itself, the tool that gets the person from point A to point B. The food the person buys at the grocery store is the electricity.
Another key component of a battery is the separator, a thin, porous membrane that, as the name implies, separates the anode and cathode electrodes while enabling the lithium ions to move from one to the other. It also prevents short-circuiting, which happens when an electric current flows down a wrong or unintended path.
What Minerals are Used in Lithium-ion Batteries?
Lithium-ion batteries usually include lithium, cobalt, manganese, nickel, and graphite. There is considerable concern about the effects of mining these minerals on local communities and landscapes. Some mines use child labour, lack safety measures to protect workers, and negatively impact the surrounding environment.
Conclusion
As there’s no overall “best” battery for all EVs. However, lithium-ion batteries currently offer the highest capabilities among available options. Semco Infratech has developed a comprehensive solution for manufacturing lithium-ion batteries with a complete assembly line. If you are someone who is looking to setup an assembly line for lithium-ion batteries, Semco is the best solution for all the machines and equipment.



